From Cosmetics to Disinfectant Products: The Compliance Gap Behind Hand Sanitizers
- Dec 15, 2025
- 3 min read
Behind a small bottle of hand sanitizer lies the regulatory boundary between cosmetics and disinfectant products. During the pandemic, China's hand sanitizer market experienced explosive growth. In 2020, sales on Alibaba platforms soared from 540 million yuan to over 2 billion yuan, a year-on-year increase of nearly 272%.
As the market continues to grow, a dazzling array of hand sanitizer products has emerged, ranging from basic cleaning to various functional options. However, for brands looking to enter the disinfectant market, that bottle of hand sanitizer may fall under two completely different regulatory systems: cosmetics or disinfectant products.
The Chinese market currently offers a wide variety of hand sanitizers, primarily categorized into two types: general cleaning hand sanitizers and functional hand sanitizers. General hand sanitizers mainly use surfactants, focusing on cleaning, stain removal, and skin care (labeled with a cosmetic-grade approval number). Functional hand sanitizers contain antibacterial or disinfectant ingredients (labeled with a disinfectant-grade approval number), providing antibacterial or bactericidal effects. The main regulatory standards for hand sanitizers in China are as follows; this article will discuss hand sanitizer compliance in conjunction with these standards:
Hand sanitizer (OB/T 2654-2013)
Hand sanitizer (GB/T 34855-2017)
Special hand sanitizer (GB 19877.1-2005)
No-rinse hand sanitizer (GB/T 43718-2024)
General requirements for hand disinfectants (GB27950-2020)
1. Hand Sanitizers (Aqueous, Gel, Foam)
Hand Sanitizers: These belong to the disinfection product category. The bottles of these products are usually labeled with the word "disinfectant" and bear a "Hygiene and Disinfection Certificate Number." The products serve a disinfection purpose, including hygienic hand disinfection and surgical hand disinfection. Products should comply with GB 27950-2020 "General Requirements for Hand Sanitizers."
No-rinse Hand Sanitizers: These products kill pathogens on the hands and do not require rinsing with water. They are primarily used for surgical hand disinfection and should comply with GB 27950-2020 "General Requirements for Hand Sanitizers."
Quick-drying Hand Sanitizers: These refer to hand sanitizers containing alcohols or skin-care ingredients. Formulations include aqueous, gel, and foam types. Products should comply with GB 27950-2020 "General Requirements for Hand Sanitizers."
Microbial Killing Indicators for Hand Sanitizers:

Toxicological indicators for hand sanitizers:

In addition, hand sanitizer formulations must not contain hormones, antibiotics, antifungal drugs or their namesake ingredients [except for disinfectant and antiseptic drugs listed in the Pharmacopoeia of the People's Republic of China (2015 edition)], or substances prohibited by the national health authorities.
2. Antibacterial (Antimicrobial) Hand Sanitizer
Antimicrobial (Antimicrobial) Hand Sanitizer: In addition to surfactants, antibacterial (antimicrobial) hand sanitizers contain antibacterial or antimicrobial ingredients. Besides cleaning, they also kill bacteria or inhibit bacterial growth.
According to the national standard GB 19877.1-2005 "Special Hand Sanitizers," the requirements for antimicrobial (antimicrobial) hand sanitizers are: when labeled as antimicrobial products, the bactericidal rate should be ≥90%; when labeled as antimicrobial products, the antimicrobial rate should be ≥50%.
• Comparison of Antimicrobial (Antimicrobial) Microbial Contamination Indicators

• Comparison of Antimicrobial (Antimicrobial) Toxicological Safety Requirements

• Comparison of Restricted and Prohibited Substances Requirements for Antimicrobial (Antimicrobial) Raw Materials
GB 15979-2024 introduces new requirements for restricted and prohibited substances in antimicrobial agents, sanitary wipes, and other disposable sanitary products with antimicrobial (antimicrobial) functions, nonwoven fabrics, textiles, or other raw materials:

3. Regular Hand Sanitizer
Regular hand sanitizer primarily serves a cleaning and decontamination function. It comes in regular and concentrated forms, but does not claim to have antibacterial or bacteriostatic effects. Current product standards include: GB/T 34855-2017 "Hand Sanitizer" and QB/T 2654-2013 "Hand Sanitizer".
Regular hand sanitizer falls under the category of general skincare and cosmetic products, offering only basic cleaning functions. It primarily bears the "Cosmetic Approval Number" mark. Antibacterial (antimicrobial) hand sanitizers...
Sensory and physicochemical indicators of regular hand sanitizer:
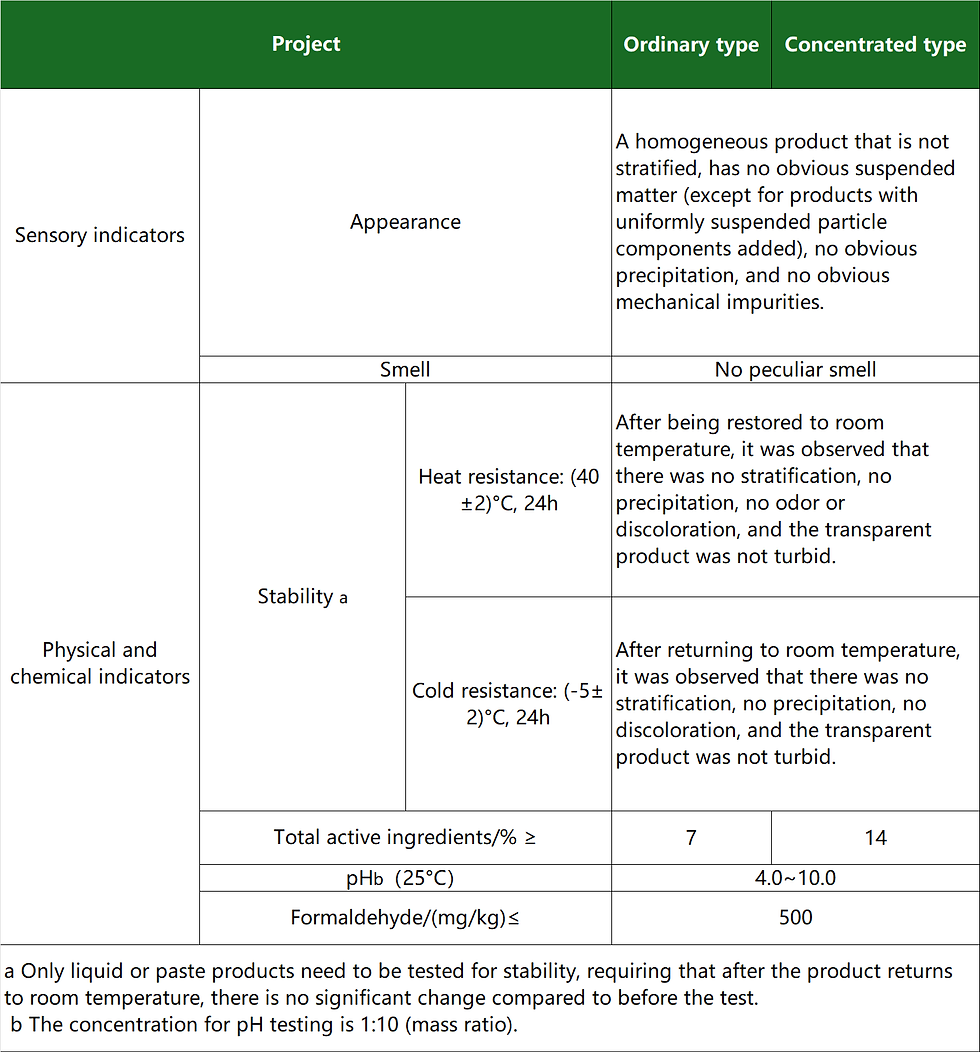
In addition, the product's microbiological indicators (total bacterial count, total mold and yeast count, thermotolerant coliforms, Staphylococcus aureus, Pseudomonas aeruginosa), limits for harmful substances (mercury, lead, arsenic, cadmium, methanol, dioxane), pH value, formaldehyde, etc., must comply with the "Cosmetic Safety Technical Specifications (2015 Edition)".
If you have any questions related to China disinfectant products registration and filing, please contact us via info@enter-co.com.
Also, you can follow us on LinkedIn for the latest cosmetic and toothpaste compliance information.



Comments